Can You Take Tramadol and Meloxicam at the Same Time
 | |
 | |
| Clinical information | |
|---|---|
| Pronunciation | tra' ma dole |
| Trade names | Ultram, Zytram, Qdolo, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695011 |
| License data |
|
| Pregnancy category |
|
| Dependence liability | Nowadays[3] |
| Routes of assistants | By rima oris, intravenous (IV), intramuscular (IM), rectal |
| Drug form | Opioid analgesic[4] |
| ATC lawmaking |
|
| Legal condition | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70–75% (by mouth), 77% (rectal), 100% (IM)[half dozen] |
| Protein binding | 20%[iii] |
| Metabolism | Liver-mediated demethylation and glucuronidation via CYP2D6 & CYP3A4[6] [vii] |
| Metabolites | O-desmethyltramadol, N-desmethyltramadol |
| Onset of activeness | Less than i hour (by mouth)[3] |
| Emptying half-life | 6.3 ± one.4 h[seven] |
| Duration of activity | 6 hours[viii] |
| Excretion | Urine (95%)[nine] |
| Identifiers | |
| IUPAC proper name
| |
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.043.912 |
| Chemic and physical information | |
| Formula | C 16 H 25 N O 2 |
| Molar mass | 263.381 g·mol−1 |
| 3D model (JSmol) |
|
| Melting point | 180 to 181 °C (356 to 358 °F) |
| SMILES
| |
| InChI
| |
| | |
Tramadol, sold under the brand proper name Ultram amidst others,[1] is an opioid pain medication used to care for moderate to moderately severe pain.[iii] When taken past mouth in an immediate-release formulation, the onset of hurting relief usually begins within an hour.[3] Information technology is besides available by injection.[10] It may be sold in combination with paracetamol (acetaminophen) or as longer-acting formulations.[three] [10]
As is typical of opioids, common side furnishings include constipation, itchiness, and nausea.[three] Serious side effects may include hallucinations, seizures, increased run a risk of serotonin syndrome, decreased alertness, and drug addiction.[3] A change in dosage may exist recommended in those with kidney or liver bug.[iii] It is non recommended in those who are at chance of suicide or in those who are pregnant.[3] [10] While non recommended in women who are breastfeeding, those who take a single dose should non generally stop breastfeeding.[11] Tramadol is converted in the liver to O-desmethyltramadol (desmetramadol), an opioid with a stronger affinity to the μ-opioid receptor.[3] [12] Tramadol is too a serotonin–norepinephrine reuptake inhibitor (SNRI).[3] [13]
Tramadol was patented in 1963 and launched nether the name "Tramal" in 1977 by the West German pharmaceutical company Grünenthal GmbH.[xiii] [fourteen] In the mid-1990s, it was canonical in the Great britain and the United States.[13] It is available equally a generic medication and marketed under many brand names worldwide.[ane] [3] In 2019, it was the 35th nigh usually prescribed medication in the United States, with more than than 19one thousand thousand prescriptions.[15] [16]
Medical uses [edit]

Generic tramadol HCl tablets marketed by Amneal Pharmaceuticals

Tramadol HCl for injection
Tramadol[17] (a schedule Four drug in the US) is used primarily to care for mild to astringent hurting, both acute and chronic.[xviii] [nineteen] There is moderate testify for employ as a 2nd-line treatment for fibromyalgia only is not FDA approved for this use;[20] however, its use is canonical for treatment of fibromyalgia as a secondary painkiller by the NHS.[21]
Its analgesic furnishings take about ane hour to come into upshot and 2 to 4 h to peak subsequently oral administration with an immediate-release formulation.[18] [19] On a dose-by-dose basis, tramadol has about one-tenth the dominance of morphine (thus 100 mg is commensurate with 10 mg morphine simply may vary) and is practically equally potent when compared with pethidine and codeine.[22] For pain moderate in severity, its effectiveness is equivalent to that of codeine at low doses, and hydrocodone at very high doses; for severe pain information technology is less effective than morphine.[18]
These painkilling effects last about vi h.[nineteen] The potency of analgesia varies considerably every bit it depends on an private's genetics. People with specific variants of CYP2D6 enzymes may not produce acceptable amounts of the active metabolite (desmetramadol) for effective hurting control.[9] [18]
Sleep medicine physicians sometimes prescribe tramadol (or other opiate medications) for refractory restless legs syndrome (RLS);[23] [24] that is, RLS that does not respond fairly to handling with commencement-line medications such as dopamine agonists (like pramipexole) or alpha-2-delta (αtwoδ) ligands (gabapentinoids), frequently due to augmentation.[25]
Contraindications [edit]
Tramadol may non provide adequate pain control for individuals with certain genetic variants of CYP2D6 enzymes as they metabolize tramadol to the inactive molecule.[ix] [eighteen] These genetic polymorphisms are not currently routinely tested for in clinical practice.[26]
Pregnancy and lactation [edit]
Tramadol'south use in pregnancy is generally avoided, equally it may crusade some reversible withdrawal effects in the newborn.[27] A modest prospective report in France establish, while an increased risk of miscarriages existed, no major malformations were reported in the newborn.[27] Its use during lactation is also generally brash against, but a small-scale trial plant that infants breastfed by mothers taking tramadol were exposed to well-nigh 2.88% of the dose the mothers were taking. No evidence of this dose harming the newborn was seen.[27]
Labor and delivery [edit]
Its use as an analgesic during labor is non advised due to its long onset of action (1 hour).[27] The ratio of the mean concentration of the drug in the fetus compared to that of the mother when it is given intramuscularly for labor pains has been estimated to be 1:94.[27]
Children [edit]
Its employ in children is more often than not advised against, although information technology may be washed nether the supervision of a specialist.[eighteen] On 21 September 2015, the FDA started investigating the safety of tramadol in use in persons under the age of 17. The investigation was initiated considering some of these people accept experienced slowed or difficult breathing.[28] The FDA lists age under 12 years old as a contraindication.[29] [thirty]
Elderly [edit]
The adventure of opioid-related adverse effects such as respiratory low, falls, cognitive impairment and sedation is increased.[xviii] Tramadol may interact with other medications and increment the take chances for adverse events.[26]
Liver and kidney failure [edit]
The drug should exist used with caution in those with liver or kidney failure, due to metabolism in the liver (to the agile molecule desmetramadol) and elimination by the kidneys.[eighteen]
Side furnishings [edit]
The most common adverse effects of tramadol include nausea, dizziness, dry out oral fissure, indigestion, abdominal pain, vertigo, vomiting, constipation, drowsiness, and headache.[31] [32] Other side furnishings may result from interactions with other medications. Tramadol has the aforementioned dose-dependent adverse effects every bit morphine including respiratory depression.[33]
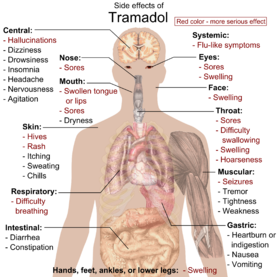
Primary side effects of tramadol: Ruby-red color denotes more than serious furnishings, requiring immediate contact with health provider.[iv]
Dependence and withdrawal [edit]
Long-term use of high doses of tramadol causes concrete dependence and withdrawal syndrome.[34] These include both symptoms typical of opioid withdrawal and those associated with serotonin–norepinephrine reuptake inhibitor (SNRI) withdrawal; symptoms include numbness, tingling, paresthesia, and tinnitus.[35] Psychiatric symptoms may include hallucinations, paranoia, extreme anxiety, panic attacks, and confusion.[36] In most cases, tramadol withdrawal will fix in 12–20 hours later the last dose, only this can vary.[35] Tramadol withdrawal typically lasts longer than that of other opioids. Seven days or more than of acute withdrawal symptoms can occur as opposed to typically 3 or 4 days for other codeine analogues.[35]
Overdose [edit]
Recognised take a chance factors for tramadol overdose include respiratory depression, habit, and seizures.[37] Naloxone only partially reverses the toxic effects of tramadol overdose and may increment the risk of seizures.[eighteen]
Deaths with tramadol overdose accept been reported and are increasing in frequency in Northern Ireland; the bulk of these overdoses involve other drugs including booze.[37] In that location were 254 tramadol-related deaths in England and Wales in 2013, and 379 in Florida in 2011.[38] [39] In 2011, 21,649 emergency room visits in the U.s.a. were related to tramadol.[40]
Interactions [edit]
Tramadol can interact with other medications with like mechanisms of action.
Tramadol acts as a serotonin-norephinephrine reuptake inhibitor and thus can interact with other serotonergic medications (selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, triptans, cough and cold medications containing dextromethorphan, herbal products containing St. John'south wort, and medications that inhibit the metabolism of serotonin, such every bit monoamine oxidase inhibitors) and, in combination, may lead to serotonin syndrome. It may also brand some serotonergic adversary anti-emetic medications (ondansetron) less effective.[41]
Tramadol also acts equally an opioid agonist and thus can increase the risk for side effects when used with other opioid analgesics (such equally morphine, pethidine, tapentadol, oxycodone, and fentanyl).[42]
Tramadol is metabolized by CYP2D6 enzymes which contribute to the metabolism of approximately 25% of all medications. Any medications with the ability to inhibit or induce these enzymes may interact with tramadol.[41]
Tramadol increases the adventure for seizures by lowering the seizure threshold. Using other medications that lower seizure threshold (such as antipsychotic medications or amphetamines), further increases this chance.[41]
Pharmacology [edit]
Mechanism of action [edit]
Tramadol induces analgesic effects through a multifariousness of unlike targets on the noradrenergic system, serotoninergic system and opioid receptors system.[43] Tramadol exists as a racemic mixture, the positive enantiomer inhibits serotonin reuptake while the negative enantiomer inhibits noradrenaline re-uptake, past binding to and blocking the transporters.[44] [8] Tramadol has also been shown to act as a serotonin releasing agent. Both enantiomers of tramadol are agonists of the μ-opioid receptor and its M1 metabolite, O-desmetramadol, is also a μ-opioid receptor agonist but is 6 times more stiff than tramadol itself.[45] All these furnishings work synergistically to induce analgesia.
| Site | Tramadol | DSMT | Species | Ref |
|---|---|---|---|---|
| MOR | i,600–12,486 2,120–8,300 ≥1,000 (EC50) | 5.iv–eighteen.vi 17 ((+)) ≥240 (ECl) | Human Rat Human | [49] [fifty] [51] [52] [53] [54] [12] |
| DOR | >10,000 57,600–100,000 | ≥2,900 690 (+)) | Human Rat | [49] [fifty] [55] [53] [52] |
| KOR | >10,000 42,700–81,000 | ≥450 1,800 (+)) | Man Rat | [49] [50] [55] [53] [52] |
| SERT | ~900 (ICl) 992–one,190 | >xx,000 (ICfifty) 2,980 ((−)) (ICl) | Human Rat | [56] [53] [12] |
| NET | 14,600 785 | ane,080 (−) (IC50) >860 (ICfifty) | Human Rat | [12] [53] [12] |
| DAT | >100,000 | >20,000 | Rat | [57] [55] |
| 5-HT1A | >20,000 | >xx,000 | Rat | [55] |
| 5-HT2A | >20,000 | >twenty,000 | Rat | [55] |
| 5-HT2C | i,000 (IC50) | 1,300 (IC50) | Rat | [58] [59] |
| 5-HT3 | >20,000 | >20,000 | Rat | [55] |
| NK1 | IA | ? | Rat | [60] [61] |
| Grand1 | >xx,000 iii,400 (ICl) | >20,000 ii,000 (IC50) | Rat Multiple | [55] [62] [63] |
| K2 | ND | ND | ND | ND |
| M3 | 1,000 (IC50) | IA | Human | [63] [64] |
| G4 | ND | ND | ND | ND |
| Mv | ND | ND | ND | ND |
| α7 | seven,400 | ND | Chicken | [65] |
| σ1 | >10,000 | ND | Rat | [46] [66] |
| σ2 | >x,000 | ND | Rat | [46] |
| NMDAR | 16,400 (ICl) | 16,500 (IC50) | Human | [67] |
| NMDAR (MK-801) | >20,000 | >20,000 | Rat | [55] |
| GABAA | >100,000 (IC50) | >100,000 (IC50) | Human being | [67] |
| GlyR | >100,000 (ICfifty) | >100,000 (ICfifty) | Man | [67] |
| TRPA1 | 100– 10,000 (SI) | 1,000– 10,000 (SI) | Human | [68] |
| TRPV1 | >10,000 (IC50) | >10,000 (IC50) | Homo | [68] [69] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | ||||
| Activeness | Value |
|---|---|
| five-HT reuptake | 1,820 |
| 5-HT release | >10,000 |
| NE reuptake | two,770 |
| NE release | >10,000 |
| DA reuptake | >10,000 |
| DA release | >10,000 |
| Values for reuptake inhibition are Grandi (nM) and for release induction are EC50 (nM). | |
Tramadol has been constitute to possess these actions:[47] [48] [44]
- Agonist of the μ-opioid receptor (MOR) and to a far lesser extent of the δ-opioid receptor (DOR) and κ-opioid receptor (KOR)
- Serotonin reuptake inhibitor (SRI) and norepinephrine reuptake inhibitor; hence, an SNRI
- Serotonin 5-HT2C receptor adversary
- Yardone and Mthree muscarinic acetylcholine receptor antagonist
- α7 nicotinic acetylcholine receptor antagonist
- NMDA receptor antagonist (very weak)
- TRPA1 inhibitor
Tramadol acts on the opioid receptors through its major active metabolite desmetramadol, which has every bit much as 700-fold higher analogousness for the MOR relative to tramadol.[12] Moreover, tramadol itself has been constitute to possess no efficacy in activating the MOR in functional activity assays, whereas desmetramadol activates the receptor with high intrinsic activity (Emax equal to that of morphine).[54] [12] [71] Every bit such, desmetramadol is exclusively responsible for the opioid effects of tramadol.[72] Both tramadol and desmetramadol have pronounced selectivity for the MOR over the DOR and KOR in terms of binding analogousness.[55] [50] [52]
Tramadol is well-established as an SRI.[47] [48] In addition, a few studies accept found that it also acts as a serotonin releasing agent (1–10 μM), like in effect to fenfluramine.[73] [74] [75] [76] The serotonin releasing effects of tramadol could be blocked by sufficiently loftier concentrations of the serotonin reuptake inhibitor 6-nitroquipazine, which is in accordance with other serotonin releasing agents such as fenfluramine and MDMA.[73] [75] [76] However, two more than recent studies failed to observe a releasing effect of tramadol at respective concentrations up to 10 and 30 μM.[77] [76] [seventy] In addition to serotonergic action, tramadol is too a norepinephrine reuptake inhibitor.[47] [48] It is not a norepinephrine releasing amanuensis.[78] [79] [80] [70] Tramadol does not inhibit the reuptake or induce the release of dopamine.[78] [70]
A positron emission tomography imaging study found that single oral 50-mg and 100-mg doses of tramadol to man volunteers resulted in 34.vii% and 50.2% respective mean occupation of the serotonin transporter (SERT) in the thalamus.[81] The estimated median constructive dose (ED50) for SERT occupancy hence was 98.1 mg, which was associated with a plasma tramadol level of about 330 ng/ml (1,300 nM).[81] The estimated maximum daily dosage of tramadol of 400 mg (100 mg q.i.d.) would result in every bit much as 78.7% occupancy of the SERT (in association with a plasma concentration of 1,220 ng/ml or 4,632 nM).[81] This is close to that of SSRIs, which occupy the SERT past 80% or more.[81]
Top plasma concentrations during treatment with clinical dosages of tramadol have generally been found to be in the range of 70 to 592 ng/ml (266–2,250 nM) for tramadol and 55 to 143 ng/ml (221–573 nM) for desmetramadol.[nineteen] The highest levels of tramadol were observed with the maximum oral daily dosage of 400 mg per mean solar day divided into one 100-mg dose every 6 hours (i.e., four 100-mg doses evenly spaced out per day).[19] [82] Some accumulation of tramadol occurs with chronic administration; peak plasma levels with the maximum oral daily dosage (100 mg q.i.d.) are about 16% college and the area-under-the-curve levels 36% college than following a single oral 100-mg dose.[19] Positron emission tomography imaging studies have reportedly found that tramadol levels are at least 4-fold higher in the encephalon than in plasma.[78] [83] Conversely, brain levels of desmetramadol "only slowly approach those in plasma".[78] The plasma protein bounden of tramadol is but 4 to xx%; hence, about all tramadol in circulation is gratuitous, thus bioactive.[84] [85] [86]
Correspondence to effects [edit]
Co-administration of quinidine, a stiff CYP2D6 enzyme inhibitor, with tramadol, a combination which results in markedly reduced levels of desmetramadol, was constitute not to significantly touch on the analgesic furnishings of tramadol in human volunteers.[12] [85] Nonetheless, other studies have plant that the analgesic effects of tramadol are significantly decreased or even absent-minded in CYP2D6 poor metabolizers.[12] [72] The analgesic effects of tramadol are only partially reversed by naloxone in man volunteers,[12] hence indicating that its opioid action is unlikely the sole factor; tramadol's analgesic effects are besides partially reversed past α2-adrenergic receptor antagonists such every bit yohimbine, the 5-HTthree receptor antagonist ondansetron, and the 5-HT7 receptor antagonists SB-269970 and SB-258719.[nineteen] [87] Pharmacologically, tramadol is similar to tapentadol and methadone in that it not only binds to the MOR, only also inhibits the reuptake of serotonin and norepinephrine[6] due to its activeness on the noradrenergic and serotonergic systems, such as its "atypical" opioid activity.[88]
Tramadol has inhibitory actions on the 5-HT2C receptor. Antagonism of 5-HT2C could exist partially responsible for tramadol's reducing consequence on depressive and obsessive–compulsive symptoms in patients with pain and co-morbid neurological illnesses.[58] v-HT2C blockade may likewise account for its lowering of the seizure threshold, every bit v-HT2C knockout mice display significantly increased vulnerability to epileptic seizures, sometimes resulting in spontaneous death. Still, the reduction of seizure threshold could be attributed to tramadol's putative inhibition of GABAA receptors at high doses (significant inhibition at 100 μM).[67] [44] In addition, desmetramadol is a high-affinity ligand of the DOR, and activation of this receptor could be involved in tramadol's ability to provoke seizures in some individuals, equally DOR agonists are well known for inducing seizures.[52]
Nausea and vomiting caused by tramadol are thought to be due to activation of the 5-HTthree receptor via increased serotonin levels.[56] In accordance, the five-HT3 receptor antagonist ondansetron tin can be used to treat tramadol-associated nausea and airsickness.[56] Tramadol and desmetramadol themselves do not bind to the 5-HT3 receptor.[56] [48]
Pharmacokinetics [edit]

Tramadol undergoes hepatic metabolism via the cytochrome P450 isozyme CYP2B6, CYP2D6, and CYP3A4, being O- and Northward-demethylated to five different metabolites. Of these, desmetramadol (O-desmethyltramadol) is the well-nigh pregnant, since it has 200 times the μ-affinity of (+)-tramadol, and furthermore has an elimination one-half-life of nine hours, compared with 6 hours for tramadol itself. Every bit with codeine, in the six% of the population who have reduced CYP2D6 activeness (hence reducing metabolism), a reduced analgesic consequence is seen. Those with decreased CYP2D6 activity require a dose increment of 30% to reach the same degree of pain relief as those with a normal level of CYP2D6 activity.[89] [90]
Phase Two hepatic metabolism renders the metabolites water-soluble, which are excreted by the kidneys. Thus, reduced doses may exist used in renal and hepatic impairment.[19]
Its book of distribution is around 306 l later on oral assistants and 203 50 after parenteral assistants.[19]
Chemistry [edit]
Tramadol is marketed as a racemic mixture of both R- and Due south-stereoisomers,[6] because the two isomers complement each other'south analgesic activities.[vi] The (+)-isomer is predominantly agile as an opiate with a higher analogousness for the µ-opiate receptor (20 times higher affinity than the (-)-isomer).[91]
Synthesis and stereoisomerism [edit]
The chemical synthesis of tramadol is described in the literature.[92] Tramadol [two-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol] has two stereogenic centers at the cyclohexane band. Thus, 2-(dimethylaminomethyl)-ane-(3-methoxyphenyl)cyclohexanol may be in 4 different configurational forms:
- (1R,2R)-isomer
- (iS,2S)-isomer
- (iR,2S)-isomer
- (aneDue south,2R)-isomer
The synthetic pathway leads to the racemate (1:1 mixture) of (1R,2R)-isomer and the (1South,2S)-isomer as the primary products. Minor amounts of the racemic mixture of the (1R,2S)-isomer and the (1Due south,iiR)-isomer are formed besides. The isolation of the (oneR,2R)-isomer and the (1S,2S)-isomer from the diastereomeric minor racemate [(1R,2S)-isomer and (aneSouthward,2R)-isomer] is realized by the recrystallization of the hydrochlorides. The drug tramadol is a racemate of the hydrochlorides of the (oneR,2R)-(+)- and the (1Southward,2S)-(−)-enantiomers. The resolution of the racemate [(1R,2R)-(+)-isomer / (1S,2S)-(−)-isomer] was described[93] employing (R)-(−)- or (Southward)-(+)-mandelic acid. This process does not find industrial awarding, since tramadol is used as a racemate, despite known different physiological effects[94] of the (oneR,2R)- and (1South,2S)-isomers, because the racemate showed higher analgesic activity than either enantiomer in animals[95] and in humans.[96]
Detection in biological fluids [edit]
Tramadol and desmetramadol may be quantified in blood, plasma or serum to monitor for abuse, confirm a diagnosis of poisoning or assist in the forensic investigation of a sudden death. Most commercial opiate immunoassay screening tests do not cantankerous-react significantly with tramadol or its major metabolites, so chromatographic techniques must exist used to notice and quantitate these substances. The concentration of desmetramadol in the claret or plasma of a person who has taken tramadol is mostly 10–20% those of the parent drug.[97] [98] [99]
Society and culture [edit]
Formulations [edit]
Available dosage forms include liquids, syrups, drops, elixirs, effervescent tablets and powders for mixing with h2o, capsules, tablets including extended-release formulations, suppositories, compounding powder, and injections.[xviii]
Patent history [edit]
The U.S. Food and Drug Administration (FDA) approved tramadol in March 1995, and an extended-release (ER) formulation in September 2005.[100] ER Tramadol was protected by US patents nos. vi,254,887[101] and 7,074,430.[102] [103] The FDA listed the patents' expiration every bit 10 May 2014.[102] However, in Baronial 2009, US Commune Court for the Commune of Delaware ruled the patents invalid, a decision upheld the following year by the Courtroom of Appeals for the Federal Excursion. Manufacture and distribution of generic equivalents of Ultram ER in the United states was therefore permitted prior to the expiration of the patents.[104]
Legal status [edit]
Constructive 18 August 2014, tramadol has been placed into Schedule 4 of the federal Controlled Substances Act in the U.s..[105] [106] Before that, some U.s.a. states had already classified tramadol as a Schedule Four controlled substance under their corresponding state laws.[107] [108] [109]
Tramadol is classified in Schedule 4 (prescription just) in Australia, rather than as a Schedule 8 Controlled Drug (Possession without potency illegal) like most other opioids.[18]
Constructive May 2008, Sweden classified tramadol as a controlled substance in the same category as codeine and dextropropoxyphene, simply allows a normal prescription to exist used.[110]
The UK classified tramadol every bit a Form C, Schedule 3 controlled drug on 10 June 2014, only exempted it from the condom custody requirement.[ citation needed ]
Misuse [edit]
Illicit apply of the drug is thought to exist a major factor in the success of the Boko Haram terrorist system.[111] [112] [113] When used at college doses, the drug "can produce like effects to heroin."[111] Said ane former member, "whenever we took tramadol, nothing mattered to the states anymore except what we were sent to do because it made us very loftier and very bold, information technology was impossible to become on a mission without taking information technology."[111] Tramadol is also used as a coping machinery in the Gaza Strip.[114] It is also driveling in the United Kingdom, inspiring the championship of the TV show Frankie Boyle'due south Tramadol Nights (2010).[115] [116]
Research [edit]
Investigational uses [edit]
- Diabetic neuropathy[117] [118]
- Antidepressant[119]
- Postherpetic neuralgia[120] [121]
- Premature ejaculation[122] [123]
- Adjunct to local anaesthesia[124]
False findings nigh sources in nature [edit]
In 2013, researchers reported that tramadol was institute in relatively high concentrations (1%+) in the roots of the African pin absorber tree (Nauclea latifolia).[125] In 2014, however, it was reported that the presence of tramadol in the tree roots was the issue of tramadol having been administered to cattle by farmers in the region:[126] tramadol and its metabolites were nowadays in the animals' excreta, which contaminated the soil around the copse. Therefore, tramadol and its mammalian metabolites were found in tree roots in the far northward of Cameroon, just not in the south where information technology is not administered to farm animals.[126]
A 2014 editorial in Lab Times online contested the notion that tramadol in tree roots was the consequence of anthropogenic contagion, stating that samples were taken from copse that grew in national parks, where livestock were forbidden; it as well quoted researcher Michel de Waard, who stated that "thousands and thousands of tramadol-treated cattle sitting around a unmarried tree and urinating there" would be required to produce the concentrations discovered.[127]
In 2015, radiocarbon analysis confirmed that the tramadol found in N.latifolia roots could non be establish-derived and was of constructed origin.[128]
Veterinary medicine [edit]
Tramadol may be used to treat postal service-operative, injury-related, and chronic (e.grand., cancer-related) pain in dogs and cats every bit well as rabbits, coatis, many pocket-size mammals including rats and flying squirrels, guinea pigs, ferrets, and raccoons.[129]
| Species | Half-life (h) for parent drug | One-half-life (h) for desmetramadol | Maximum plasma concentration (ng/mL) for parent drug | Maximum plasma concentration (ng/mL) for desmetramadol |
|---|---|---|---|---|
| Camel | iii.two (IM), 1.iii (IV) | – | 0.44 (4) | – |
| Cat | 3.forty (oral), 2.23 (IV) | 4.82 (oral), iv.35 (Iv) | 914 (oral), 1323 (Four) | 655 (oral), 366 (IV) |
| Dog | i.71 (oral), 1.80 (IV), ii.24 (rectal) | 2.18 (oral), 90-5000 (IV) | 1402.75 (oral) | 449.xiii (oral), 90–350 (Four) |
| Ass | four.ii (oral), i.v (IV) | – | 2817 (oral) | – |
| Goat | 2.67 (oral), 0.94 (Four) | – | 542.9 (oral) | – |
| Horses | 1.29–1.53 (Iv), 10.one (oral) | iv (oral) | 637 (Iv), 256 (oral) | 47 (oral) |
| Llama | 2.54 (IM), 2.12 (Four) | vii.73 (IM), x.four (Iv) | 4036 (4), 1360 (IM) | 158 (IV), 158 (IM) |
References [edit]
- ^ a b c "Tramadol". Drugs.com . Retrieved 22 December 2018.
- ^ "Tramadol Utilize During Pregnancy". Drugs.com. 14 October 2019. Retrieved 7 Feb 2020.
- ^ a b c d eastward f thousand h i j k l 1000 "Tramadol Hydrochloride". The American Gild of Wellness-System Pharmacists. Retrieved 1 December 2014.
- ^ a b "Tramadol". MedlinePlus. American Society of Health-System Pharmacists. ane September 2008. Retrieved 29 September 2009.
- ^ "List of nationally authorised medicinal products" (PDF). European Medicines Bureau. 28 January 2021. Retrieved 21 July 2021.
{{cite spider web}}: CS1 maint: url-status (link) - ^ a b c d east Brayfield, A, ed. (13 December 2013). "Tramadol Hydrochloride". Martindale: The Complete Drug Reference. Pharmaceutical Printing. Retrieved 5 April 2014.
- ^ a b "Ultram, Ultram ER (tramadol) dosing, indications, interactions, adverse effects, and more than". Medscape Reference. WebMD. Retrieved 28 November 2013.
- ^ a b Dayer P, Desmeules J, Collart 50 (1997). "[Pharmacology of tramadol]". Drugs. 53 (Suppl 2): 18–24. doi:10.2165/00003495-199700532-00006. PMID 9190321. S2CID 46970093.
- ^ a b c "Australian Characterization: Tramadol Sandoz 50 mg capsules" (PDF). TGA eBusiness Services. 4 November 2011. Retrieved half dozen April 2014.
- ^ a b c British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. pp. 447–448. ISBN978-0857112989.
- ^ "Tramadol Pregnancy and Breastfeeding Warnings". Drugs.com . Retrieved five September 2016.
- ^ a b c d e f 1000 h i j Raffa RB, Buschmann H, Christoph T, Eichenbaum G, Englberger Westward, Flores CM, et al. (July 2012). "Mechanistic and functional differentiation of tapentadol and tramadol". Expert Opinion on Pharmacotherapy. 13 (10): 1437–1449. doi:ten.1517/14656566.2012.696097. PMID 22698264. S2CID 24226747.
- ^ a b c Leppert W (Nov–December 2009). "Tramadol equally an analgesic for mild to moderate cancer pain". Pharmacological Reports. 61 (6): 978–992. doi:10.1016/s1734-1140(09)70159-eight. PMID 20081232.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 528. ISBN9783527607495.
- ^ "The Top 300 of 2019". ClinCalc . Retrieved xvi October 2021.
- ^ "Tramadol – Drug Usage Statistics". ClinCalc . Retrieved sixteen October 2021.
- ^ "Tramadol Hydrochloride Tablet Uses, Dosage, Benefits, Side Effects, Warnings, Free Advice About Tramadol 2021". 19 November 2021. Retrieved 9 December 2021.
- ^ a b c d east f yard h i j k Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN978-0-9805790-9-3.
- ^ a b c d e f g h i Grond S, Sablotzki A (2004). "Clinical pharmacology of tramadol". Clinical Pharmacokinetics. 43 (13): 879–923. doi:10.2165/00003088-200443130-00004. PMID 15509185. S2CID 32347667.
- ^ MacLean AJ, Schwartz TL (May 2015). "Tramadol for the treatment of fibromyalgia". Expert Review of Neurotherapeutics. 15 (5): 469–475. doi:10.1586/14737175.2015.1034693. PMID 25896486. S2CID 26613022.
- ^ "Treatment – Fibromyalgia (NHS)". NHS. 20 February 2019. Archived from the original on 4 September 2020. Retrieved 4 September 2020.
- ^ Lee CR, McTavish D, Sorkin EM (August 1993). "Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic backdrop, and therapeutic potential in acute and chronic pain states". Drugs. 46 (2): 313–340. doi:10.2165/00003495-199346020-00008. PMID 7691519.
- ^ Silber MH, Becker PM, Buchfuhrer MJ, Earley CJ, Ondo WG, Walters As, Winkelman JW (January 2018). "The Advisable Use of Opioids in the Treatment of Refractory Restless Legs Syndrome". Mayo Clinic Proceedings. 93 (one): 59–67. doi:x.1016/j.mayocp.2017.11.007. PMID 29304922.
In summary, a number of opioid medications in low dose appear effective in refractory RLS. The risks of opioid utilize are relatively low, taking into account the much lower doses used for RLS compared with those in patients with pain syndromes. Equally long as reasonable precautions are taken, the chance-benefit ratio is acceptable and opioids should not be unreasonably withheld from such patients.
- ^ Winkelmann J, Allen RP, Högl B, Inoue Y, Oertel W, Salminen AV, et al. (July 2018). "Treatment of restless legs syndrome: Evidence-based review and implications for clinical practice (Revised 2017)§". Motion Disorders. 33 (7): 1077–1091. doi:10.1002/mds.27260. PMID 29756335. S2CID 21669996.
- ^ Lipford MC, Silber MH (Dec 2012). "Long-term use of pramipexole in the management of restless legs syndrome". Slumber Medicine. 13 (x): 1280–1285. doi:10.1016/j.sleep.2012.08.004. PMID 23036265.
For the purposes of this study, augmentation was divers as earlier onset, increased severity, [increased] duration, or new anatomic distribution of RLS symptoms during treatment.
- ^ a b Miotto K, Cho AK, Khalil MA, Blanco Chiliad, Sasaki JD, Rawson R (January 2017). "Trends in Tramadol: Pharmacology, Metabolism, and Misuse". Anesthesia and Analgesia. 124 (1): 44–51. doi:10.1213/ANE.0000000000001683. PMID 27861439. S2CID 24224625.
- ^ a b c d due east Bloor Chiliad, Paech MJ, Kaye R (April 2012). "Tramadol in pregnancy and lactation". International Journal of Obstetric Anesthesia. 21 (2): 163–167. doi:10.1016/j.ijoa.2011.10.008. PMID 22317891.
- ^ "FDA Drug Safety Communication: FDA evaluating the risks of using the pain medicine tramadol in children aged 17 and younger". FDA. FDA Drug Prophylactic and Availability. Retrieved 21 September 2015.
- ^ Commissioner, Office of the. "Press Announcements – FDA statement from Douglas Throckmorton, M.D., deputy center manager for regulatory programs, Center for Drug Evaluation and Research, on new warnings almost the use of codeine and tramadol in children & nursing mothers". www.fda.gov . Retrieved 21 April 2017.
- ^ "FDA Drug Safety Advice: FDA restricts employ of prescription codeine pain and coughing medicines and tramadol pain medicines in children; recommends confronting utilise in breastfeeding women". Nutrient and Drug Assistants. nine February 2019.
- ^ Langley PC, Patkar AD, Boswell KA, Benson CJ, Schein JR (January 2010). "Agin event profile of tramadol in contempo clinical studies of chronic osteoarthritis pain". Current Medical Inquiry and Stance. 26 (1): 239–251. doi:10.1185/03007990903426787. PMID 19929615. S2CID 20703694.
- ^ Keating GM (2006). "Tramadol sustained-release capsules". Drugs. 66 (two): 223–230. doi:x.2165/00003495-200666020-00006. PMID 16451094. S2CID 22620947.
- ^ ""Weak" opioid analgesics. Codeine, dihydrocodeine and tramadol: no less risky than morphine". Prescrire International. 25 (168): 45–50. February 2016. PMID 27042732.
- ^ "Withdrawal syndrome and dependence: tramadol as well". Prescrire International. 12 (65): 99–100. June 2003. PMID 12825576.
- ^ a b c Epstein DH, Preston KL, Jasinski DR (July 2006). "Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol". Biological Psychology. 73 (1): 90–99. doi:10.1016/j.biopsycho.2006.01.010. PMC2943845. PMID 16497429.
- ^ Senay EC, Adams EH, Geller A, Inciardi JA, Muñoz A, Schnoll SH, et al. (Apr 2003). "Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur". Drug and Alcohol Dependence. 69 (iii): 233–241. CiteSeerX10.i.1.524.5426. doi:10.1016/S0376-8716(02)00321-half dozen. PMID 12633909.
- ^ a b Randall C, Crane J (March 2014). "Tramadol deaths in Northern Ireland: a review of cases from 1996 to 2012". Periodical of Forensic and Legal Medicine. 23: 32–36. doi:10.1016/j.jflm.2014.01.006. PMID 24661703.
- ^ White M. "Tramadol Deaths in the Uk" (pdf_e). Public Wellness England.
- ^ Fauber J (22 Dec 2013). "Killing Hurting: Tramadol the 'Condom' Drug of Abuse".
- ^ Scheck J (19 October 2016). "Tramadol: The Opioid Crisis for the rest of the World". The Wall Street Journal. Dow Jones & Co. Retrieved 4 January 2019.
- ^ a b c Miotto K, Cho AK, Khalil MA, Blanco Yard, Sasaki JD, Rawson R (January 2017). "Trends in Tramadol: Pharmacology, Metabolism, and Misuse". Anesthesia and Analgesia. 124 (1): 44–51. doi:x.1213/1.0000000000001683. PMID 27861439. S2CID 24224625.
- ^ Pasternak, G.W. Md, PhD (March 2012). "Preclinical Pharmacology and Opioid Combinations". Pain Medicine. 13 (one): 4–11. doi:ten.1111/j.1526-4637.2012.01335.x. PMID 22420604.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hitchings A, Lonsdale D, Burrage D, Baker Due east (2015). Summit 100 drugs : clinical pharmacology and applied prescribing. Churchill Livingstone Elsevier. pp. 168–169. ISBN978-0-7020-5516-4.
- ^ a b c Vazzana 1000, Andreani T, Fangueiro J, Faggio C, Silva C, Santini A, et al. (March 2015). "Tramadol hydrochloride: pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems". Biomedicine & Pharmacotherapy. 70: 234–238. doi:10.1016/j.biopha.2015.01.022. PMID 25776506.
- ^ "Tramadol". world wide web.drugbank.ca.
- ^ a b c Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Plan (PDSP). University of North Carolina at Chapel Hill and the United States National Constitute of Mental Health. Retrieved 14 August 2017.
- ^ a b c d Minami K, Uezono Y, Ueta Y (March 2007). "Pharmacological aspects of the effects of tramadol on G-poly peptide coupled receptors". Journal of Pharmacological Sciences. 103 (3): 253–260. doi:ten.1254/jphs.cr0060032. PMID 17380034.
- ^ a b c d e Minami K, Ogata J, Uezono Y (Oct 2015). "What is the main mechanism of tramadol?". Naunyn-Schmiedeberg's Athenaeum of Pharmacology. 388 (ten): 999–1007. doi:10.1007/s00210-015-1167-5. PMID 26292636. S2CID 9066672.
- ^ a b c Wentland MP, Lou R, Lu Q, Bu Y, VanAlstine MA, Cohen DJ, Bidlack JM (January 2009). "Syntheses and opioid receptor bounden properties of carboxamido-substituted opioids". Bioorganic & Medicinal Chemistry Messages. 19 (ane): 203–208. doi:10.1016/j.bmcl.2008.10.134. PMID 19027293.
- ^ a b c d Shen Q, Qian Y, Huang X, Xu Ten, Li W, Liu J, Fu Westward (Apr 2016). "Discovery of Stiff and Selective Agonists of δ Opioid Receptor by Revisiting the "Bulletin-Accost" Concept". ACS Medicinal Chemistry Letters. 7 (4): 391–396. doi:10.1021/acsmedchemlett.5b00423. PMC4834657. PMID 27096047.
- ^ Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, et al. (April 2011). "Compatible assessment and ranking of opioid μ receptor binding constants for selected opioid drugs". Regulatory Toxicology and Pharmacology. 59 (three): 385–390. doi:ten.1016/j.yrtph.2010.12.007. PMID 21215785.
- ^ a b c d east Potschka H, Friderichs East, Löscher W (September 2000). "Anticonvulsant and proconvulsant effects of tramadol, its enantiomers and its M1 metabolite in the rat kindling model of epilepsy". British Journal of Pharmacology. 131 (2): 203–212. doi:10.1038/sj.bjp.0703562. PMC1572317. PMID 10991912.
- ^ a b c d e Codd EE, Shank RP, Schupsky JJ, Raffa RB (September 1995). "Serotonin and norepinephrine uptake inhibiting activity of centrally interim analgesics: structural determinants and role in antinociception". The Journal of Pharmacology and Experimental Therapeutics. 274 (3): 1263–1270. PMID 7562497.
- ^ a b Gillen C, Haurand M, Kobelt DJ, Wnendt S (August 2000). "Affinity, authorisation and efficacy of tramadol and its metabolites at the cloned man mu-opioid receptor". Naunyn-Schmiedeberg's Archives of Pharmacology. 362 (two): 116–121. doi:10.1007/s002100000266. PMID 10961373. S2CID 10459734.
- ^ a b c d eastward f m h i Frink MC, Hennies HH, Englberger West, Haurand M, Wilffert B (November 1996). "Influence of tramadol on neurotransmitter systems of the rat brain". Arzneimittel-Forschung. 46 (11): 1029–1036. PMID 8955860.
- ^ a b c d Barann M, Urban B, Stamer U, Dorner Z, Bönisch H, Brüss M (February 2006). "Furnishings of tramadol and O-demethyl-tramadol on homo v-HT reuptake carriers and human v-HT3A receptors: a possible mechanism for tramadol-induced early on emesis". European Journal of Pharmacology. 531 (one–3): 54–58. doi:ten.1016/j.ejphar.2005.11.054. PMID 16427041.
- ^ Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL (January 1992). "Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an 'singular' opioid analgesic". The Journal of Pharmacology and Experimental Therapeutics. 260 (i): 275–285. PMID 1309873.
- ^ a b Ogata J, Minami Grand, Uezono Y, Okamoto T, Shiraishi M, Shigematsu A, Ueta Y (May 2004). "The inhibitory effects of tramadol on 5-hydroxytryptamine blazon 2C receptors expressed in Xenopus oocytes". Anesthesia and Analgesia. 98 (five): 1401–six, table of contents. doi:10.1213/01.ANE.0000108963.77623.A4. PMID 15105221. S2CID 41718739.
- ^ Horishita T, Minami K, Uezono Y, Shiraishi M, Ogata J, Okamoto T, Shigematsu A (2006). "The tramadol metabolite, O-desmethyl tramadol, inhibits 5-hydroxytryptamine type 2C receptors expressed in Xenopus Oocytes". Pharmacology. 77 (2): 93–99. doi:10.1159/000093179. PMID 16679816. S2CID 23775035.
- ^ Okamoto T, Minami K, Uezono Y, Ogata J, Shiraishi Grand, Shigematsu A, Ueta Y (July 2003). "The inhibitory furnishings of ketamine and pentobarbital on substance p receptors expressed in Xenopus oocytes". Anesthesia and Analgesia. 97 (1): 104–x, tabular array of contents. doi:x.1213/01.ANE.0000066260.99680.xi. PMID 12818951. S2CID 35809206.
- ^ Minami Yard, Yokoyama T, Ogata J, Uezono Y (2011). "The tramadol metabolite O-desmethyl tramadol inhibits substance P-receptor functions expressed in Xenopus oocytes". Journal of Pharmacological Sciences. 115 (3): 421–424. doi:10.1254/jphs.10313sc. PMID 21372504.
- ^ Shiraishi M, Minami K, Uezono Y, Yanagihara Due north, Shigematsu A (October 2001). "Inhibition by tramadol of muscarinic receptor-induced responses in cultured adrenal medullary cells and in Xenopus laevis oocytes expressing cloned M1 receptors". The Journal of Pharmacology and Experimental Therapeutics. 299 (1): 255–260. PMID 11561087.
- ^ a b Nakamura M, Minami Yard, Uezono Y, Horishita T, Ogata J, Shiraishi M, et al. (July 2005). "The furnishings of the tramadol metabolite O-desmethyl tramadol on muscarinic receptor-induced responses in Xenopus oocytes expressing cloned M1 or M3 receptors". Anesthesia and Analgesia. 101 (1): 180–6, table of contents. doi:x.1213/01.1.0000154303.93909.A3. PMID 15976229. S2CID 23861688.
- ^ Shiga Y, Minami G, Shiraishi Yard, Uezono Y, Murasaki O, Kaibara G, Shigematsu A (November 2002). "The inhibitory furnishings of tramadol on muscarinic receptor-induced responses in Xenopus oocytes expressing cloned M(3) receptors". Anesthesia and Analgesia. 95 (v): 1269–73, table of contents. doi:x.1097/00000539-200211000-00031. PMID 12401609. S2CID 39621215.
- ^ Shiraishi Thou, Minami K, Uezono Y, Yanagihara N, Shigematsu A, Shibuya I (May 2002). "Inhibitory effects of tramadol on nicotinic acetylcholine receptors in adrenal chromaffin cells and in Xenopus oocytes expressing alpha 7 receptors". British Journal of Pharmacology. 136 (2): 207–216. doi:x.1038/sj.bjp.0704703. PMC1573343. PMID 12010769.
- ^ Sánchez-Fernández C, Montilla-García Á, González-Cano R, Nieto FR, Romero L, Artacho-Cordón A, et al. (Jan 2014). "Modulation of peripheral μ-opioid analgesia by σ1 receptors". The Journal of Pharmacology and Experimental Therapeutics. 348 (1): 32–45. doi:x.1124/jpet.113.208272. PMID 24155346. S2CID 6884854.
- ^ a b c d Hara Chiliad, Minami K, Sata T (May 2005). "The effects of tramadol and its metabolite on glycine, gamma-aminobutyric acidA, and North-methyl-D-aspartate receptors expressed in Xenopus oocytes". Anesthesia and Analgesia. 100 (five): 1400–1405. doi:10.1213/01.ANE.0000150961.24747.98. PMID 15845694. S2CID 35342038.
- ^ a b Miyano K, Minami K, Yokoyama T, Ohbuchi G, Yamaguchi T, Murakami S, et al. (Apr 2015). "Tramadol and its metabolite m1 selectively suppress transient receptor potential ankyrin 1 activity, but not transient receptor potential vanilloid one action". Anesthesia and Analgesia. 120 (4): 790–798. doi:x.1213/ANE.0000000000000625. PMID 25642661. S2CID 8082654.
- ^ Marincsák R, Tóth BI, Czifra G, Szabó T, Kovács 50, Bíró T (June 2008). "The analgesic drug, tramadol, acts as an agonist of the transient receptor potential vanilloid-1". Anesthesia and Analgesia. 106 (half-dozen): 1890–1896. doi:10.1213/ane.0b013e318172fefc. PMID 18499628. S2CID 45854233.
- ^ a b c d Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry. 6 (17): 1845–1859. doi:10.2174/156802606778249766. PMID 17017961.
- ^ Minami Yard, Sudo Y, Miyano Grand, Irish potato RS, Uezono Y (June 2015). "µ-Opioid receptor activation by tramadol and O-desmethyltramadol (M1)". Journal of Anesthesia. 29 (iii): 475–479. doi:10.1007/s00540-014-1946-z. PMID 25394761. S2CID 7091648.
- ^ a b Coller JK, Christrup LL, Somogyi AA (February 2009). "Role of active metabolites in the apply of opioids". European Journal of Clinical Pharmacology. 65 (two): 121–139. doi:10.1007/s00228-008-0570-y. PMID 18958460. S2CID 9977741.
- ^ a b Driessen B, Reimann Due west (January 1992). "Interaction of the primal analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitro". British Periodical of Pharmacology. 105 (1): 147–151. doi:x.1111/j.1476-5381.1992.tb14226.x. PMC1908625. PMID 1596676.
- ^ Bamigbade TA, Davidson C, Langford RM, Stamford JA (September 1997). "Deportment of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (five-HT) efflux and uptake in the rat dorsal raphe nucleus". British Journal of Anaesthesia. 79 (3): 352–356. doi:10.1093/bja/79.3.352. PMID 9389855. S2CID 15630689.
- ^ a b Reimann Due west, Schneider F (May 1998). "Induction of v-hydroxytryptamine release by tramadol, fenfluramine and reserpine". European Periodical of Pharmacology. 349 (ii–3): 199–203. doi:ten.1016/S0014-2999(98)00195-2. PMID 9671098.
- ^ a b c Gobbi Yard, Moia 1000, Pirona 50, Ceglia I, Reyes-Parada M, Scorza C, Mennini T (September 2002). "p-Methylthioamphetamine and 1-(grand-chlorophenyl)piperazine, two non-neurotoxic 5-HT releasers in vivo, differ from neurotoxic amphetamine derivatives in their mode of activity at 5-HT nerve endings in vitro". Periodical of Neurochemistry. 82 (6): 1435–1443. doi:x.1046/j.1471-4159.2002.01073.x. hdl:10533/173421. PMID 12354291. S2CID 13397864.
- ^ Gobbi Thousand, Mennini T (April 1999). "Release studies with rat brain cortical synaptosomes indicate that tramadol is a 5-hydroxytryptamine uptake blocker and not a v-hydroxytryptamine releaser". European Journal of Pharmacology. 370 (1): 23–26. doi:10.1016/s0014-2999(99)00123-5. PMID 10323276.
- ^ a b c d Driessen B, Reimann W, Giertz H (March 1993). "Furnishings of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro". British Periodical of Pharmacology. 108 (3): 806–811. doi:10.1111/j.1476-5381.1993.tb12882.10. PMC1908052. PMID 8467366.
- ^ Reimann W, Hennies HH (June 1994). "Inhibition of spinal noradrenaline uptake in rats past the centrally acting analgesic tramadol". Biochemical Pharmacology. 47 (12): 2289–2293. doi:10.1016/0006-2952(94)90267-4. PMID 8031323.
- ^ Halfpenny DM, Callado LF, Hopwood SE, Bamigbade TA, Langford RM, Stamford JA (December 1999). "Effects of tramadol stereoisomers on norepinephrine efflux and uptake in the rat locus coeruleus measured past real time voltammetry". British Journal of Anaesthesia. 83 (vi): 909–915. doi:ten.1093/bja/83.6.909. PMID 10700792. S2CID 17830312.
- ^ a b c d Ogawa 1000, Tateno A, Arakawa R, Sakayori T, Ikeda Y, Suzuki H, Okubo Y (June 2014). "Occupancy of serotonin transporter by tramadol: a positron emission tomography study with [11C]DASB". The International Journal of Neuropsychopharmacology. 17 (6): 845–850. doi:x.1017/S1461145713001764. PMID 24423243.
- ^ "Tramadol Dosage Guide with Precautions".
- ^ Tao Q, Stone DJ, Borenstein MR, Codd EE, Coogan TP, Desai-Krieger D, et al. (April 2002). "Differential tramadol and O-desmethyl metabolite levels in brain vs. plasma of mice and rats administered tramadol hydrochloride orally". Journal of Clinical Chemist's shop and Therapeutics. 27 (two): 99–106. doi:x.1046/j.1365-2710.2002.00384.x. PMID 11975693. S2CID 42370985.
- ^ Gibson TP (July 1996). "Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl". The American Periodical of Medicine. 101 (1A): 47S–53S. doi:10.1016/s0002-9343(96)90035-2. PMID 8764760.
- ^ a b Dayer P, Collart L, Desmeules J (1994). "The pharmacology of tramadol". Drugs. 47 (Suppl 1): three–7. doi:x.2165/00003495-199400471-00003. PMID 7517823. S2CID 33474225.
- ^ Nobilis M, Kopecký J, Kvetina J, Chládek J, Svoboda Z, Vorísek Five, et al. (March 2002). "High-operation liquid chromatographic determination of tramadol and its O-desmethylated metabolite in blood plasma. Application to a bioequivalence study in humans". Journal of Chromatography A. 949 (1–ii): eleven–22. doi:10.1016/S0021-9673(01)01567-nine. PMID 11999728.
- ^ Yanarates O, Dogrul A, Yildirim V, Sahin A, Sizlan A, Seyrek Thou, et al. (March 2010). "Spinal 5-HT7 receptors play an important office in the antinociceptive and antihyperalgesic effects of tramadol and its metabolite, O-Desmethyltramadol, via activation of descending serotonergic pathways". Anesthesiology. 112 (3): 696–710. doi:10.1097/ALN.0b013e3181cd7920. PMID 20179508. S2CID 11913166.
- ^ Micó JA, Ardid D, Berrocoso Due east, Eschalier A (July 2006). "Antidepressants and pain". Trends in Pharmacological Sciences. 27 (vii): 348–354. doi:x.1016/j.tips.2006.05.004. PMID 16762426.
- ^ Leppert W (2011). "CYP2D6 in the metabolism of opioids for mild to moderate pain". Pharmacology. 87 (5–6): 274–285. doi:x.1159/000326085. PMID 21494059.
- ^ Samer CF, Lorenzini KI, Rollason V, Daali Y, Desmeules JA (June 2013). "Applications of CYP450 testing in the clinical setting". Molecular Diagnosis & Therapy. 17 (3): 165–184. doi:10.1007/s40291-013-0028-5. PMC3663206. PMID 23588782.
- ^ "Tramadol Hydrochloride 50mg Capsules". U.k. Electronic Medicines Compendium. January 2016. Retrieved 16 March 2017.
- ^ Pharmaceutical Substances, Axel Kleemann, Jürgen Engel, Bernd Kutscher and Dieter Reichert, 4. ed. (2000) two volumes, Thieme-Verlag Stuttgart (Germany), p. 2085 bis 2086, ISBN 978-1-58890-031-ix; since 2003 online with biannual actualizations.
- ^ Zynovy Z, Meckler H (2000). "A Practical Process for the Resolution of (+)- and (−)-Tramadol". Organic Process Research & Evolution. 4 (4): 291–294. doi:10.1021/op000281v.
- ^ Burke D, Henderson DJ (April 2002). "Chirality: a blueprint for the hereafter". British Journal of Anaesthesia. 88 (4): 563–576. doi:x.1093/bja/88.4.563. PMID 12066734.
- ^ Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL, et al. (October 1993). "Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol". The Periodical of Pharmacology and Experimental Therapeutics. 267 (1): 331–340. PMID 8229760.
- ^ Grond Southward, Meuser T, Zech D, Hennig U, Lehmann KA (September 1995). "Analgesic efficacy and safety of tramadol enantiomers in comparison with the racemate: a randomised, double-blind study with gynaecological patients using intravenous patient-controlled analgesia". Hurting. 62 (three): 313–320. doi:10.1016/0304-3959(94)00274-I. PMID 8657431. S2CID 34150137.
- ^ Karhu D, El-Jammal A, Dupain T, Gaulin D, Bouchard S (September 2007). "Pharmacokinetics and dose proportionality of 3 Tramadol Contramid OAD tablet strengths". Biopharmaceutics & Drug Disposition. 28 (half dozen): 323–330. doi:10.1002/bdd.561. PMID 17575561. S2CID 22720069.
- ^ Tjäderborn M, Jönsson AK, Hägg S, Ahlner J (December 2007). "Fatal unintentional intoxications with tramadol during 1995-2005". Forensic Science International. 173 (2–3): 107–111. doi:ten.1016/j.forsciint.2007.02.007. PMID 17350197.
- ^ Baselt, R. (2017) Disposition of Toxic Drugs and Chemicals in Human, 11th edition, Biomedical Publications, Seal Embankment, CA, pp. 2185-2188, ISBN 978-0-692-77499-ane.
- ^ McCarberg B (June 2007). "Tramadol extended-release in the direction of chronic pain". Therapeutics and Clinical Take a chance Management. 3 (3): 401–410. PMC2386353. PMID 18488071.
- ^ Usa patent 6254887, Miller RB, Leslie ST, Malkowska ST, Smith KJ, Wimmer Southward, Winkler H, Hahn U, Prater DA, "Controlled Release Tramadol", issued 3 July 2001
- ^ a b FDA AccessData entry for Tramadol Hydrochloride. Retrieved 17 Baronial 2009.
- ^ US patent 7074430, Miller RB, Malkowska ST, Wimmer South, Hahn U, Leslie ST, Smith KJ, Winkler H, Prater DA, "Controlled Release Tramadol Tramadol Formulation", issued 11 July 2006
- ^ Purdue Pharma Prods. L.P. v. Par Pharm., Inc. , 377 Fed.Appx. 978 (Fed. Cir. 2010).
- ^ "DEA controls tramadol as a schedule Four controlled substance constructive August 18, 2014". FDA Police Weblog. two July 2014.
- ^ "Federal Registrar" (PDF). gpo.gov.
- ^ "TRAMADOL (Trade Names: Ultram, Ultracet)". Drug Enforcement Administration (February 2011)
- ^ "Tennessee News: Tramadol and Carisoprodol Now Classified Schedule Four". National Association of Boards of Chemist's (8 June 2011). Retrieved on 26 December 2012.
- ^ "State of Ohio Board of Chemist's" (PDF). Pharmacy.ohio.gov. eighteen August 2014. Archived from the original (PDF) on 29 Dec 2016. Retrieved 8 November 2016.
- ^ "Substansen tramadol nu narkotikaklassad på samma sätt som kodein och dextropropoxifen" (in Swedish). Lakemedelsverket. xiv May 2008. Retrieved 12 August 2019.
- ^ a b c "If you lot take Tramadol away, you make Boko Haram weak". African Arguments. xv March 2019. Retrieved eighteen March 2019.
- ^ "Drugs for state of war: Opioid abuse in Due west Africa". BBC News . Retrieved eighteen March 2019.
- ^ "The Dangerous Opioid from Republic of india". www.csis.org . Retrieved 18 March 2019.
- ^ Berger, Miriam (vii January 2019). "Gaza's Opioid Problem". The Nation. ISSN 0027-8378. Retrieved 18 March 2019.
- ^ Winstock AR, Borschmann R, Bell J (September 2014). "The non-medical use of tramadol in the UK: findings from a large community sample". International Journal of Clinical Practice. 68 (9): 1147–1151. doi:ten.1111/ijcp.12429. PMID 24734958. S2CID 21883884.
- ^ "Tramadol Corruption & Addiction Causes". UK Addiction Treatment Centres. eight Baronial 2018.
- ^ Harati Y, Gooch C, Swenson Grand, Edelman Due south, Greene D, Raskin P, et al. (June 1998). "Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy". Neurology. 50 (6): 1842–1846. doi:10.1212/WNL.50.half dozen.1842. PMID 9633738. S2CID 45709223.
- ^ Harati Y, Gooch C, Swenson Grand, Edelman SV, Greene D, Raskin P, et al. (2000). "Maintenance of the long-term effectiveness of tramadol in treatment of the pain of diabetic neuropathy". Journal of Diabetes and Its Complications. 14 (2): 65–lxx. doi:ten.1016/S1056-8727(00)00060-X. PMID 10959067.
- ^ Barber J (April 2011). "Examining the use of tramadol hydrochloride as an antidepressant". Experimental and Clinical Psychopharmacology. 19 (2): 123–130. doi:10.1037/a0022721. PMID 21463069.
- ^ Göbel H, Stadler T (1997). "[Treatment of mail-canker zoster pain with tramadol. Results of an open pilot written report versus clomipramine with or without levomepromazine]". Drugs (in French). 53 (Suppl 2): 34–39. doi:10.2165/00003495-199700532-00008. PMID 9190323. S2CID 46986791.
- ^ Boureau F, Legallicier P, Kabir-Ahmadi Yard (July 2003). "Tramadol in post-herpetic neuralgia: a randomized, double-bullheaded, placebo-controlled trial". Pain. 104 (1–two): 323–331. doi:10.1016/S0304-3959(03)00020-four. PMID 12855342. S2CID 42979548.
- ^ Wu T, Yue X, Duan X, Luo D, Cheng Y, Tian Y, Wang Thou (September 2012). "Efficacy and safety of tramadol for premature ejaculation: a systematic review and meta-analysis". Urology. eighty (iii): 618–624. doi:10.1016/j.urology.2012.05.035. PMID 22840860.
- ^ Wong BL, Malde S (January 2013). "The use of tramadol "on-demand" for premature ejaculation: a systematic review". Urology. 81 (1): 98–103. doi:ten.1016/j.urology.2012.08.037. PMID 23102445.
- ^ Ryan T, Hodge A, Holyoak R, Vlok R, Melhuish T, Binks Yard, et al. (July 2019). "Tramadol every bit an adjunct to intra-articular local anaesthetic infiltration in knee joint arthroscopy: a systematic review and meta-assay". ANZ Journal of Surgery. 89 (7–8): 827–832. doi:10.1111/ans.14920. PMID 30684306. S2CID 59275648.
- ^ Boumendjel A, Sotoing Taïwe Yard, Ngo Bum Due east, Chabrol T, Beney C, Sinniger 5, et al. (November 2013). "Occurrence of the synthetic analgesic tramadol in an African medicinal plant". Angewandte Chemie. 52 (45): 11780–11784. doi:10.1002/anie.201305697. PMID 24014188.
- ^ a b Kusari S, Tatsimo SJ, Zühlke S, Talontsi FM, Kouam SF, Spiteller M (November 2014). "Tramadol--a true natural product?". Angewandte Chemie. 53 (45): 12073–12076. doi:10.1002/anie.201406639. PMID 25219922.
- ^ Who Actually did it First? Nature or a Pharmacist?, in Lab Times online; by Nicola Chase; published 22 September 2014; retrieved 21 Nov 2015
- ^ Kusari S, Tatsimo SJ, Zühlke Southward, Spiteller M (January 2016). "Synthetic Origin of Tramadol in the Surround". Angewandte Chemie. 55 (i): 240–243. doi:x.1002/anie.201508646. PMID 26473295.
- ^ a b Souza MJ, Cox SK (Jan 2011). "Tramadol use in zoologic medicine". The Veterinary Clinics of North America. Exotic Animal Practise. fourteen (1): 117–130. doi:10.1016/j.cvex.2010.09.005. PMID 21074707.
Further reading [edit]
- Dean Fifty (2015). "Tramadol Therapy and CYP2D6 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Data (NCBI). PMID 28520365. Bookshelf ID: NBK315950.
External links [edit]
- "Tramadol". Drug Data Portal. U.S. National Library of Medicine.
- "Tramadol hydrochloride". Drug Data Portal. U.S. National Library of Medicine.
steinkewerelf1974.blogspot.com
Source: https://en.wikipedia.org/wiki/Tramadol
Post a Comment for "Can You Take Tramadol and Meloxicam at the Same Time"